Tailor-made stainless steel solutions for the highest pharmaceutical requirements
Sterile precision. Made in Germany.
Weiss Apparatebau stands for certified quality and maximum process reliability in highly sensitive areas of the pharmaceutical industry.
Our components and systems are manufactured in accordance with GMP guidelines and the requirements of DIN EN ISO 3834-3 as well as AD 2000 HP0 – perfectly suited for sterile production environments with the highest hygiene standards.
Certified quality for pharmaceutical processes
With state-of-the-art CNC, welding, and laser technology as well as validated production, we guarantee:
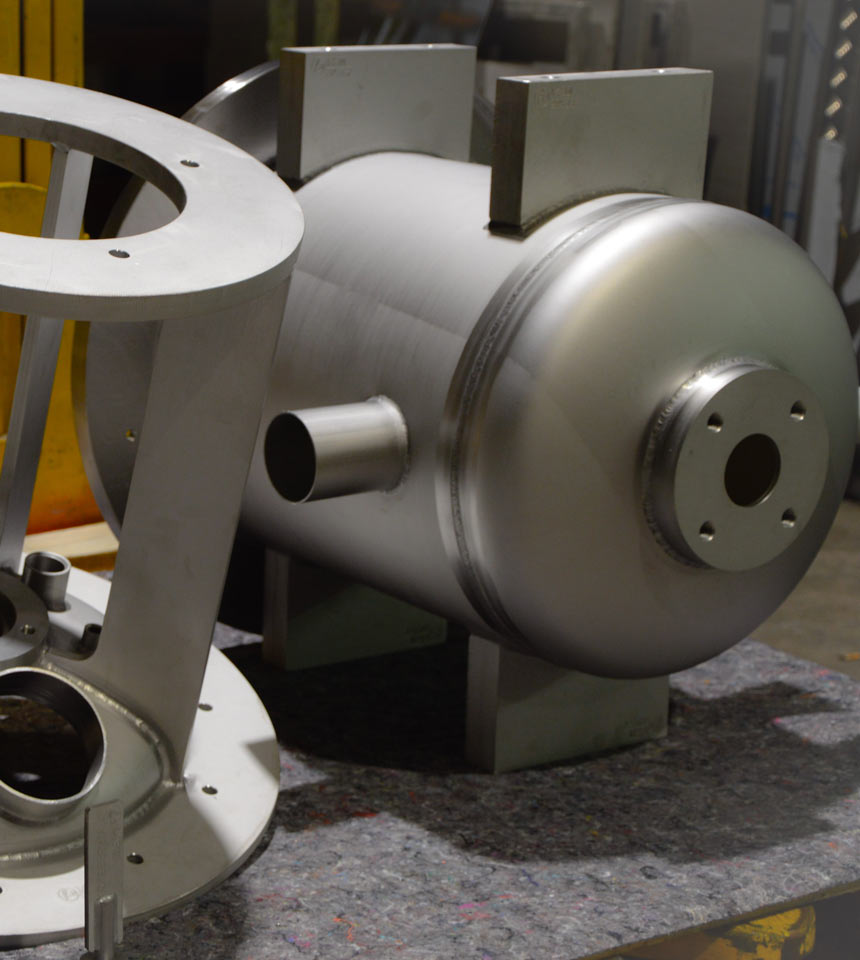

Why stainless steel technology is so critical in the pharmaceutical industry

In pharmaceutical production, extremely strict requirements apply – even the slightest deviations can compromise the effectiveness of a drug or cause contamination.
The stainless steel technology used must therefore not only be precise, but above all hygienic (hygienic design) and GMP-compliant.
Weiss Apparatebau understands these strict requirements and delivers components precisely designed for cleanrooms and demanding hygienic environments.

Tested quality in every production step
Certified processes for maximum product safety
Weiss Apparatebau manufactures according to strict quality guidelines – from material selection and welding to final inspection. Every step is documented, tested, and traceable. Certifications according to AD 2000 HP0 and DIN EN ISO 3834-3 confirm our quality promise. This ensures solutions our pharmaceutical industry customers can rely on 100% – even during audits.


Custom stainless steel technology for the highest pharma standards.
Safety, proven, certified
Sterile process environments
Weiss Apparatebau understands the high demands of the pharmaceutical industry. Our solutions meet strict standards for hygiene, safety, and traceability – perfectly suited for sensitive production environments.
Seamless documentation
For every solution, we provide a comprehensive documentation package: from material certificates and welding records to test reports – audit-ready and fully traceable down to the last batch.
GMP & EHEDG compliant
Our systems and components are manufactured in accordance with GMP guidelines and hygienic design principles (EHEDG) – ensuring contamination-free processes and validated safety in the pharmaceutical sector.
Reliability & Safety
We deliver systems you can rely on: highly precise, fail-safe, and validatable – ensuring stable processes and products that consistently meet the highest quality standards.
Insights into certified sterile technology
Answers to your most important questions – directly from the experts

